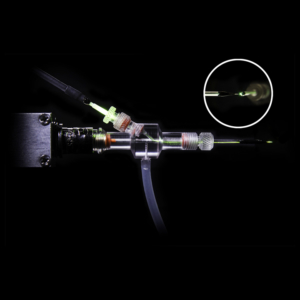
Overview
The HSPC-2-SB is an easy-to-use device for generating arbitrary pressure waveforms for the study of mechanosensitive ion channels during patch clamp recording. It is also used to stimulate the inner hair cells in the ear canal. The device consists of a control unit and a small headstage.
If you study mechanosensitive channels or stimulate inner hair cells, then you need ALA’s High Speed Pressure Clamp, HSPC-2-SB. The HSPC-2-SB is the only commercial instrument that can generate reproducible and rapid pressure/vacuum steps. Adding the HSPC-2-SB and the PV-Pump accessory to any patch clamp rig creates a complete system for biophysical studies in this important area.
Last Updated on March 31, 2025
Details
The 2nd generation High Speed Pressure Clamp (HSPC-2SB) device is a closed loop pressure-control servo optimized to deliver vacuum and pressure at zero average flow. Although the device is targeted at patch clamp applications, response characteristics are fully adjustable for use in other experimental environments. It features a steady state pressure range of ±200 mmHg, hardware to prevent water entering the control valve, an external command input and a buffered output pressure monitor. Command response has been optimized for steps as large as +/-200 mmHg. Pressure rise time is amplitude dependent and varies from 6 to 8 ms for a 20 mmHg step to 15 ms to 20 ms for a 200 mmHg step approximately. Fall time is also amplitude dependent and varies from 6 to 8 ms for a 20 mmHg step to 15 ms to 20 ms for a 200 mmHg step approximately. There is an amplitude independent delay of 1 ms on both the rising and falling edges of the response. System noise measured at the pressure sensor output is +/-0.5 mmHg peak to peak.
HSPC-2-SB System Highlights
• Pressure and vacuum partitioned by small headstage that easily mounts near amplifier probe
• Simple connection to electrode holder transmits pressure/vacuum pulses
• Command input of 20mV/mmHg sets pressure
• Pressure output of 20mV/mmHg signal monitor or from LCD display
• Moisture sensor prolongs life of headstage
• Compatible with all major patch clamp hardware/software
• Improves consistency of establishing gigaseals and whole-cell configuration
Last Updated on March 31, 2025
Specifications
| Max. Input Pressure/Vacuum |
+/-7psi; 362mmHg |
| Standard Output Pressure/Vacuum Range |
+/-200mmHg |
| Noise |
+/-10mV; +/-1mmHg |
| Power |
9V dc, 1A fuse |
| Controller |
2.9lbs/1.32kg – 8.5”/21.6cm x 7.5”/19cm x 4”/10cm |
| Headstage |
0.5lbs/0.23kg – 3.75”/9.5cm x 1.75”/4.4cm x 1.75”/4.4cm |
| Typical Speed of Response |
0 to 100mmHg jump in 12ms: 0 to 100% settling time
Command Input 20mV/mmHg |
| Command Input |
20mV/mmHg |
| Monitor Output |
20mV/mmHg |
| Set Point Control (Holding Pressure/Vacuum offset control) |
+/-200mmHg |
| Moisture Alarm |
Capacitance liquid detection sensor to protect valve |
P-V Pump:
| Voltage |
115V AC or 230V AC |
| Maximum Airflow |
0.12 cfm / 3 l/min |
| Maximum Vacuum |
17.5 in”Hg / 592 mbar |
| Frequency |
60 Hz / 50 Hz |
| Noise Leve |
40 dB(A) Max. @ 1 meter |
| Recognition |
UL / CE |
| Dimensions |
32cmx10cmx15cm ht. |
| Weight |
3.4Kg |
Last Updated on March 31, 2025
Ordering
| HSPC-2-SB |
Complete pressure clamp system includes controller, piezo valve headstage and cable, misc. tubing, and fittings |
| PV-Pump |
Pressure / vacuum pumps with output tubing |
Last Updated on March 31, 2025